Chromatography is used extensively in chemical, biomedical, industrial, research, and other laboratories for analytical and quality control purposes. There are numerous types of chromatography methods and each is based on the material being analyzed as well as other chemical properties.
While chromatography methods range in scope, complexity, and methodologies, a well-defined underlying thread does exist: Every chromatography method aims to separate a material into its different components in a way so each component can be identified and quantified. This is why chromatography is widely known as the “science of separation.”
“Chromatography plays a crucial role in analytical science, offering a reliable and efficient method for separating and analyzing complex mixtures. Its ability to deliver precise and quantifiable results makes it a fundamental tool across various industries, including pharmaceuticals, biotechnology, and environmental science.”
- Joshua Crolle, Director of Business Development at Pipette.com
The chromatographic methods that laboratories use today can be divided into two main categories: liquid chromatography and gas chromatography. In this article, we will explore the basics of both as well as how they’re commonly used by today’s analytical chemists.
What is Chromatography?
Chromatography is the scientific process of separating a mixture into two or more components, usually for the purpose of identifying and quantifying the different components and compounds within the mixture.
Separating a material, compound, or mixture into its various components allows scientists to understand the material they’re testing, identify what’s in it, and also determine how much of each component is in the sample.
Ways chromatography is used to in different industries and scientific areas include:
- Environmental testing. Chromatography is a key analytical tool used in environmental testing laboratories for testing water, soil, and air samples for contaminants.
- Quality control. Chromatography helps pharmaceutical and supplement manufacturers assess the quality of their products before releasing them for sale. It is also useful for monitoring production processes in chemical plants.
- Research. Chromatography helps researchers in numerous industries including the pharmaceutical, energy, manufacturing, and medical sectors.
A Real-World Example of Chromatography
Let’s consider a water sample taken from a lake where a gas spill occurred; the lake is a source of drinking water for the area’s residents. Local authorities need to determine if the water is safe for drinking and for swimming. A water sample is collected from the spill area and taken to an approved environmental testing laboratory.
Using chromatographic methods, technicians at the environmental testing lab can identify what contaminants are present in the water as well as how much of each contaminant is present in the water. This information is used to determine if the lake’s water quality is sufficient for residents to drink from.
|
More articles from the Pipette.com blog we think you’ll enjoy: |
How Does Chromatography Work?
Earlier, we discussed the underlying thread among all chromatography methods is they are used to separate a mixture into different parts. However, that’s not the only commonality. Another key commonality is that every chromatography method separates a mixture using a mobile phase and a stationary phase.
What is a Stationary Phase?
The stationary phase is the medium, or surface, where a mixture is applied and will help facilitate separating a mixture into its different components. The stationary phase stays in place and the sample moves through it.
Stationary phase material can vary in composition, particle size, and polarity. Common materials include glass, silica, alumina, silicone based oils, and more.
In both liquid and gas chromatography, a stationary phase is called a “column”.
What is a Mobile Phase?
The mobile phase is the medium that transports a sample through the stationary phase. It enables the mixture to separate into its components.
Mobile phase can be liquid or gas. In gas chromatography, the mobile phase is gas with the most common being nitrogen, helium, or hydrogen. A GC’s mobile phase is often referred to as the “carrier gas".
In liquid chromatography, the mobile phase is a liquid. Common liquids used for a mobile phase include Acetonitrile, Methanol, or other suitable solutions.
The Two Types of Chromatography Methods: GC and LC
Gas Chromatography (GC) and Liquid Chromatography (LC) are the two main categories of chromatography methods under the chromatography umbrella. Each is used extensively for chemical analysis and research.
Gas Chromatography (GC)
Gas Chromatography is used to separate volatile compounds, such as oil and gas samples, paint, or pesticides. Gas is used as the mobile phase, carrying the sample through the GC column.
Liquid Chromatography (LC)
Liquid Chromatography utilizes a liquid as the mobile phase, making it incredibly versatile and suitable for a broad spectrum of samples, including large molecules and those that are not easily vaporized. It’s common in biochemistry and pharmaceutical labs.
Modern use of liquid chromatography is typically with High-Performance Liquid Chromatography (HPLC). There are five primary types of HPLC that include:
- Reversed-Phase chromatography
- Normal Phase chromatography
- Ion Exchange chromatography
- Size Exclusion chromatography
- Affinity chromatography
What Equipment is Needed for the Different Types of Chromatography?
The mobile phase and stationary phase are just one piece of many in a modern chromatography set up. Here’s what you need for Gas Chromatography (GC) and Liquid Chromatography (LC).
Equipment for Gas Chromatography (GC)
Gas Supply and Carrier Gas
A stable gas supply is vital for any GC analysis, as the gas is the crucial mobile phase of the analysis. Inert gasses are required for this purpose and helium is often a top choice as a carrier gas.
Injector
An injector is used to inject a sample into the GC system. Modern GC systems have auto-injectors that will automatically pick up a sample vial and carry it to the injection port where an injection needle will automatically draw some of the sample and inject it into the system.
Column
The column, also known as the stationary phase, is the medium where separation occurs. Compounds in the sample interact differently with this coating, leading to their separation.
Some components are more “drawn” to the column material than others. The greater attraction a component has for the column, the longer it will take to elute (travel along and leave) a column. Some compounds move faster than others, which is how they separate from each other.
Oven
Heat is essential to a GC analysis. Heat will help separations occur more efficiently and more effectively. The oven’s job is to maintain a stable temperature, or stable temperature gradient, that is a critical factor for consistent separation and reliable results.
Detectors
Separating a mixture is only half of the puzzle. The other half is analyzing each component using a detector. Examples of detectors used for a GC analysis include Flame Ionization Detectors (FID), Ultra-Violet (UV) detectors, Mass Spectrometers (MS). There are several other types of detectors. Conduct your own research to determine which one is best for your application.
Equipment for Liquid Chromatography (LC)
Solvent Reservoir
The solvent reservoir pertains to the bottles used to hold the mobile phase solutions needed for a LC analysis. They are typically 2L - 4L in size and are placed at the top of an LC apparatus.
Pump
The pump of an HPLC pumps the mobile phase through the system and column. It carries the sample in and out of the column. A pump must be consistent and ensure a steady flow of solvent and sample through the system is maintained—this is crucial for consistent chromatography results.
Injector
The injector of an HPLC is generally the same as one used in a GC setup. If a sample’s stability is temperature dependent, the auto injector could be temperature controlled. Just as with the GC injector, a robotic arm will pick up a sample and carry it to the injector port where a needle automatically draws a sample from the vial and injects it into the system for analysis.
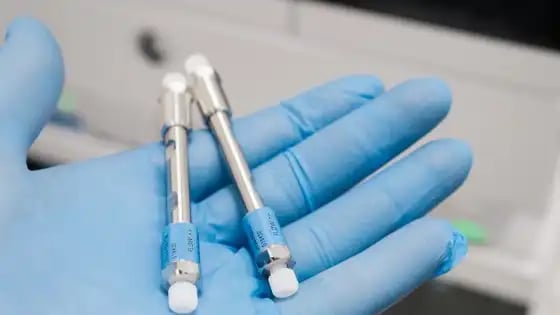
Column
The HPLC column, aka the stationary phase of the HPLC analysis, has the same function as the column on a GC instrument. There are different types of columns that enable separations using different chemical principles.
In an analysis, once the sample enters the column, its different components will separate based on the chemical properties of the sample and its affinity for the column material.
Detector
Detectors in an HPLC, such as UV spectrophotometers, work to identify and quantify the sample components after separation, completing the chromatography process. Other types of detectors include Mass Spectrometer (MS), Fluorescence, Refractive Index (RI), and more.
Essential Supplies for Chromatography From Pipette.com
Chromatography analysis requires specialized supplies such as chromatography vials, columns, inert vial inserts, secure vial tops, and more. These are critical components that ensure the results of an analysis are accurate and reliable.
When shopping for supplies for your HPLC chromatography assays, look no further than Pipette.com. We have a wide variety of columns, HPLC accessories, sample preparation supplies from top brands you love.
Explore our selection of quality lab supplies at Pipette.com.


